Featured
Quantum Theory Of An Atom
Quantum Theory Of An Atom. R h is constant and n is the principle quantum # this yields an atomic model that looks like: 5 d orbitals ⇒ 10 electrons.
The new era in physics started in 1900 with a young german physicist named max planck. Gained or lost by the atom. An electron that is as close to the nucleus as it can is in its lowest energy level, the farther an electron is from the.
While Analyzing The Data On Radiation Emitted By Solids.
It took longer time to realize and accept that the properties of atoms and molecules are not governed by the same physical laws as larger objects. Gained or lost by the atom. R h is constant and n is the principle quantum # this yields an atomic model that looks like:
The Quantum Mechanical Model Of The Atom Uses Complex Shapes Of Orbitals (Sometimes Called Electron Clouds ), Volumes Of Space In Which There Is Likely To Be An Electron.
Atomic theory is the scientific theory that matter is composed of particles called atoms. Introduction to the quantum mechanical model of the atom: Along with the standing wave, there are points of zero displacements, known as nodes.
The New Era In Physics Started In 1900 With A Young German Physicist Named Max Planck.
7 f orbitals ⇒ 14 electrons. Each distance equal to a certain quantity of energy that an electron can have. The spatial orientation of an orbital ml page number :
They Exist As A Fuzzy Cloud Of Negative Charge Around The Nucleus, Instead Of As A Particle Located At A Single Point.
The quantum theory of atoms in molecules, developed by richard f. Quantum theory describes matter as acting both as a particle and as a wave. As is well documented, the atomic and group properties predicted by qtaim agree with the additive group contributions.
The Size Of An Orbital N 4.
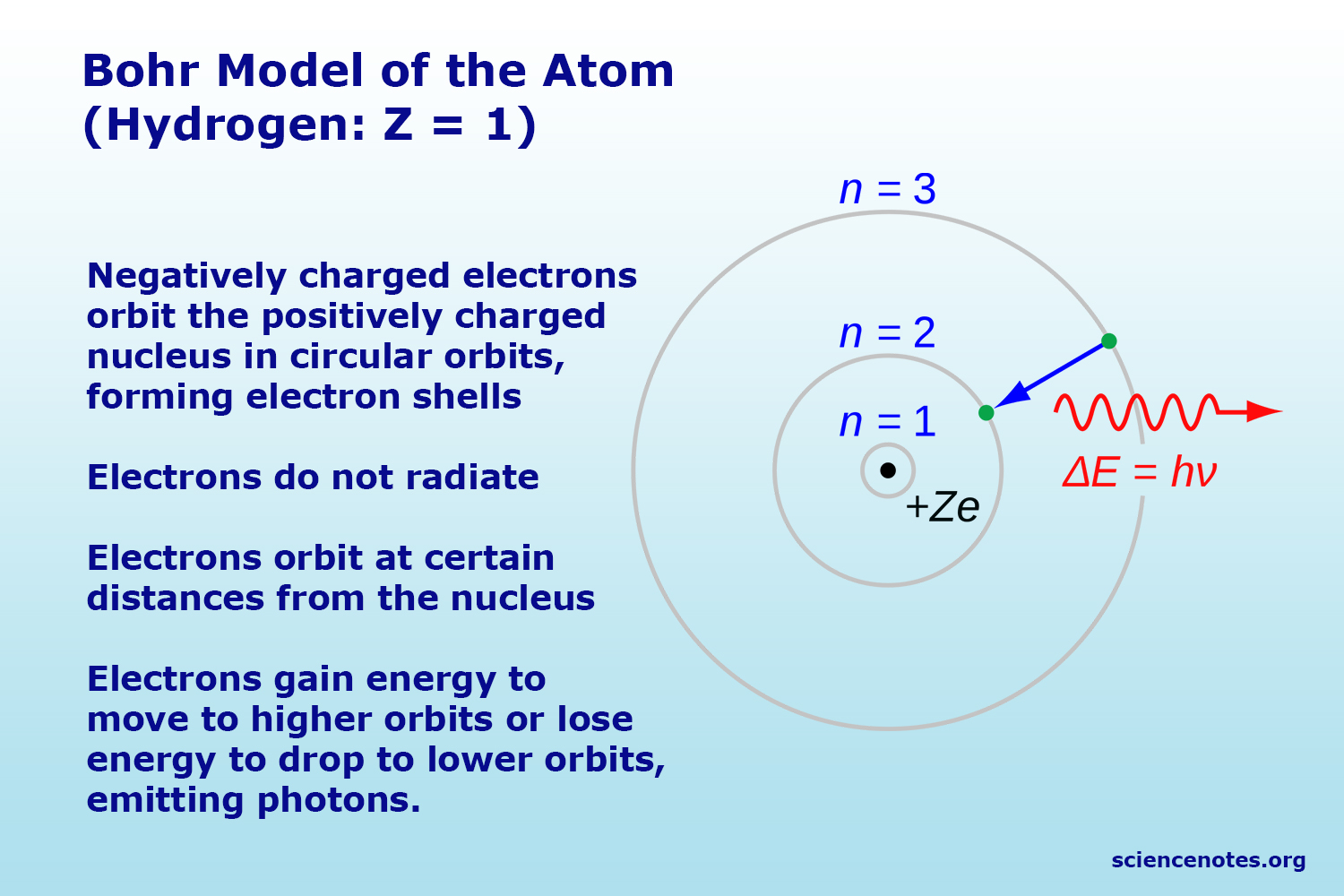
Bohr’s model of the atom electrons can be only at a certain distances from the nucleus. An atomic orbital can hold a maximum of 2 electrons. While elements of the theory.
Comments
Post a Comment